Key Findings
Lewis Acid Zeolite Catalysts Enable Enhanced C3+ Selectivity
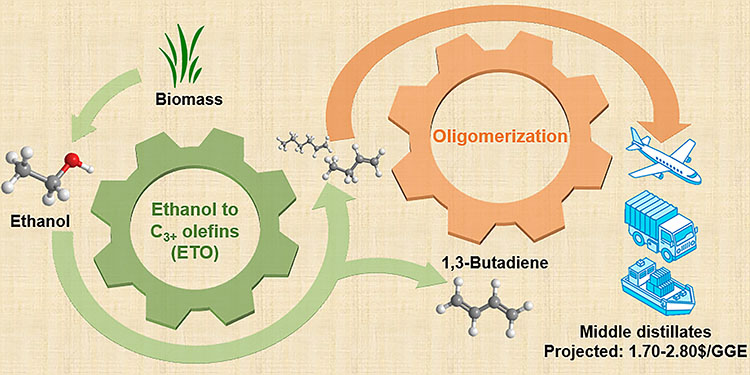
When catalyzed by Lewis acid zeolites, the pathway for forming carbon-carbon bonds exhibits much higher C3+ olefin selectivity compared to other direct ethanol-to-butene-rich olefin approaches. This is possible because such catalysts minimize ethanol dehydration to ethelyne through a unique active site combination. These catalysts also avoid significant carbon-carbon cleavage and over-hydrogenation of olefins, thereby preventing the formation of CO2 and light paraffins, respectively.
C3+ Olefins Can Be Upgraded to Longer-Chain Hydrocarbons Over Several Solid Acid Catalysts
C3+ olefins obtained from this one-step ethanol conversion process are further oligomerized to longer-chain hydrocarbons over several solid acid catalysts, including Amberlyst-15, Amberlyst-36, and CT275. Findings suggest that gasoline, jet, and diesel cuts can be adjusted by using different oligomerization catalysts.
The Approach for Making Middle Distillate Fuels From Renewable Ethanol Could Have Cost Advantages
Compared to two-step ethanol to butene-rich olefin processes, this one-step approach can reduce ethanol upgrading costs by as much as 42% ($0.60/gasoline gallon equivalent (GGE) vs $1.04/GGE). When using a pure ethanol feed, the baseline ethanol upgrading cost is $0.60/GGE, with capital expenses contributing 27% and operations 73% of the total cost.
Related ChemCatBio Capabilities
More Sources on This Topic
Isolated Metal Sites in Cu–Zn–Y/Beta for Direct and Selective Butene-Rich C3+ Olefin Formation from Ethanol, ACS Catalysis (2021)
Selective Butene Formation in Direct Ethanol-to-C3+-Olefin Valorization over Zn–Y/Beta and Single-Atom Alloy Composite Catalysts Using In Situ-Generated Hydrogen, ACS Catalysis (2021)
Selective Conversion of Bio-Derived Ethanol to Renewable BTX Over Ga-ZSM-5, Green Chemistry (2017)
Catalytic Conversion of Biomass-Derived Ethanol to Liquid Hydrocarbon Blendstock: Effect of Light Gas Recirculation, Energy & Fuels (2016)