Branched Hydrocarbons From Syngas and Carbon Dioxide in a Single Reactor
ChemCatBio 2023 Technology Brief
This study combines commercially available catalysts with a catalyst developed by ChemCatBio to intensify and streamline the conversion of renewable syngas and carbon dioxide (CO2) to hydrocarbons suited for making sustainable aviation fuel.
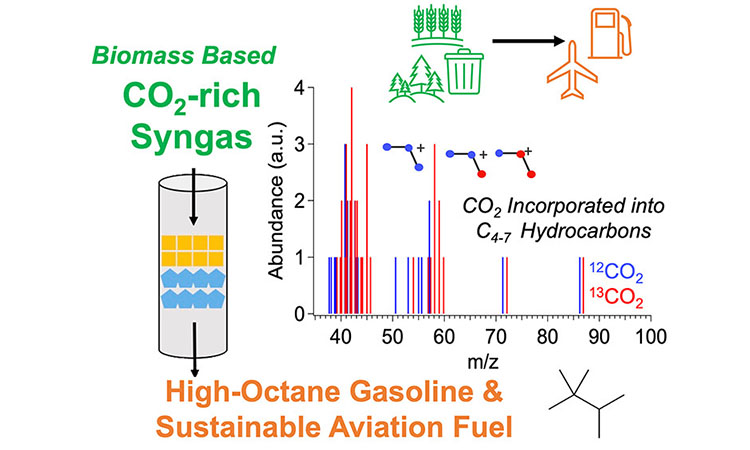
This research demonstrates a one-step approach to convert syngas—a mixture of carbon monoxide (CO), hydrogen, and CO2—to hydrocarbons. Renewable syngas, created during biomass gasification, contains about 20% CO2 after a standard reforming step. Using a single reactor to convert this CO2 alongside other syngas compounds provides a unique opportunity to increase carbon efficiency from gasification-based processes. Such a one-reactor approach can reduce capital and operating expenses compared to traditional, multi-reactor routes for making high-value, high-octane gasoline and sustainable aviation fuel.
Direct Conversion of Renewable CO2-Rich Syngas to High-Octane Hydrocarbons in a Single Reactor
ACS Catalysis, 2022
Dan Ruddy
[email protected]
Key Findings
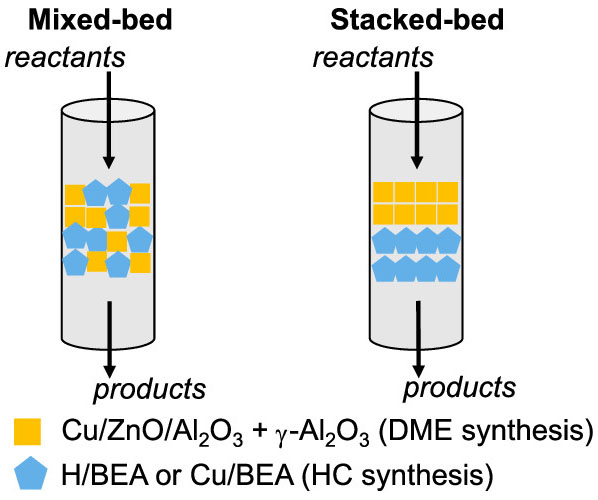
Stacked-Bed Copper-Modified Beta Zeolite (Cu/BEA) Catalyst Performs Best
Mixed catalyst beds provide high CO conversions and C4+ yields, but they also result in undesirable high CO2 selectivity above 45%. Stacked catalyst beds, on the other hand, increase C4+ selectivity and decrease CO2 selectivity by about 15%. Cu/BEA catalyst performed best in this initial assessment. These selectivity data indicate two advantages of positioning the hydrocarbon synthesis catalyst (BEA zeolite or Cu/BEA) downstream of the syngas-activating catalysts:
- It favors the hydrocarbon pool methylation chemistry, improving selectivity to C4+ products.
- It simultaneously minimizes the water–gas shift side reaction, decreasing undesirable CO2 selectivity.
Product selectivity over mixed and stacked catalyst beds
More Cu/BEA in the Catalyst Composition Increases Hydrocarbon Yield
Data show that hydrocarbon yield can be controlled by changing the mass ratio of catalysts in the reactor. By increasing Cu/BEA content in syngas-activating catalysts, C4+ yields increased from 13.7% to 19.1% to 23.5%. Meanwhile, yields of undesirable dimethyl ether decreased from 15.8% to 5.5%. Together, these results confirm the ability to control product yield simply by changing the composition of the catalyst.
Loading Level:
Yields of C4+ hydrocarbons and dimethyl ether as a function of Cu/BEA catalyst composition
Isotope Experiments Indicate CO2 Conversion to Hydrocarbon Products
Mass spectrometry and isotope tracing of syngas conversion reactions confirms the conversion of CO2 into hydrocarbons. When CO2 labeled with carbon-13 is fed through the reactor, mass fragment peaks shift to a mass-to-charge ratio of +1 or greater. This indicates CO2 activation and incorporation into desired hydrocarbon products like isobutane, triptane, and others.
Mass spectrometry of two abundant hydrocarbon products (isobutane and triptane) during syngas-to-hydrocarbons reactions. Orange data show results of 13C-labelled CO2. Blue lines show results of CO2 without isotopic labeling.